Poster (P39)
Role of Covalency in Influencing the Magnetic Anisotropy in Uranium Molecular Magnets
Sourav Dey, Gopalan Rajaraman*
Department of Chemistry, Indian Institute of Technology Bombay
Email: rajaraman[at]chem.iitb.ac.in
Theoretical investigation on actinide based nanomagnets is of paramount interest in the field of molecular magnetism because of difficulty to understand its electronic structure using other probes. The magnetic property of actinide can be fine tuned by modulating the ligand field just like lanthanides, however due to its larger covalency, the effects are expected to be more dramatic. It has the best of both worlds combining the strong anisotropy like that found in lanthanides and tuneable metal-ligand covalency like that are attributed to transition metals complexes.1These inherently enhance the exchange coupling constants in polymetalllic Uranium based SMM. Not only SMMs but also SIMs of actinides displays better magnetic properties than analogues lanthanides.2Theoretical study on actinide based SMM is rare due to the complicated nature of its magnetic relaxation mechanism and the associated electronic structure. The complex electronic structure arises from the open shell 5f electrons and strong relativistic effects which makes the actinide chemistry very interesting. Due to the comparable energy of the 7p, 7s, 6d, 5f orbitals, the ground state electronic structure is inherently multiconfigurational in nature and to get a complete understanding of the electronic structure those orbital needs to take into consideration. Here using ab initio CASSCF calculations will be utilized to understand the nature of magnetic anisotropy. The choice of the active space is crucial in ab initio study of actinides and we have taken into consideration the 5f and 6d orbitals in our active space. The magnetic exchange between {3d-ff} pair has been explored by DFT methodology. To reveal the physical nature of bonding and agostic interaction we have also performed quantum theory of atoms in molecules (QTAIM) and natural bond orbital (NBO) analysis.
|
|
|
|
|
|
|
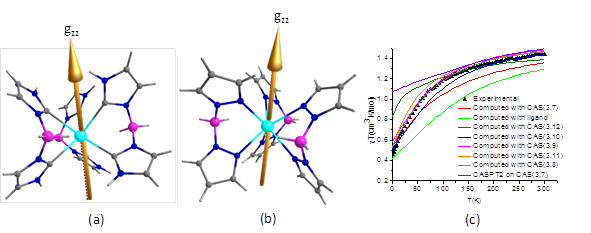
Figure 1: (a) Anisotropy axis of U(BcMe)3 (b) Anisotropy axis of U(BpMe)3 (c) The computed magnetic susceptibility with varying the size of the active space.
References:
1. Chatelain, L.; Walsh, J. P.; Pcaut, J.; Tuna, F.; Mazzanti, M. Angew. Chem.2014, 126, 13652.
2. Meihaus, K. R.; Minasian, S. G.; Lukens Jr, W. W.; Kozimor, S. A.; Shuh, D. K.; Tyliszczak, T.; Long, J. R. J. Am. Chem. Soc.2014, 136, 6056